.gif)

-

-
2025 β - N - 乙酰葡萄糖胺苷酶;Ludger 货号:E-GL01;Β-N-Acetylglucosaminidase;参考价RMB 5524元(具体询价)
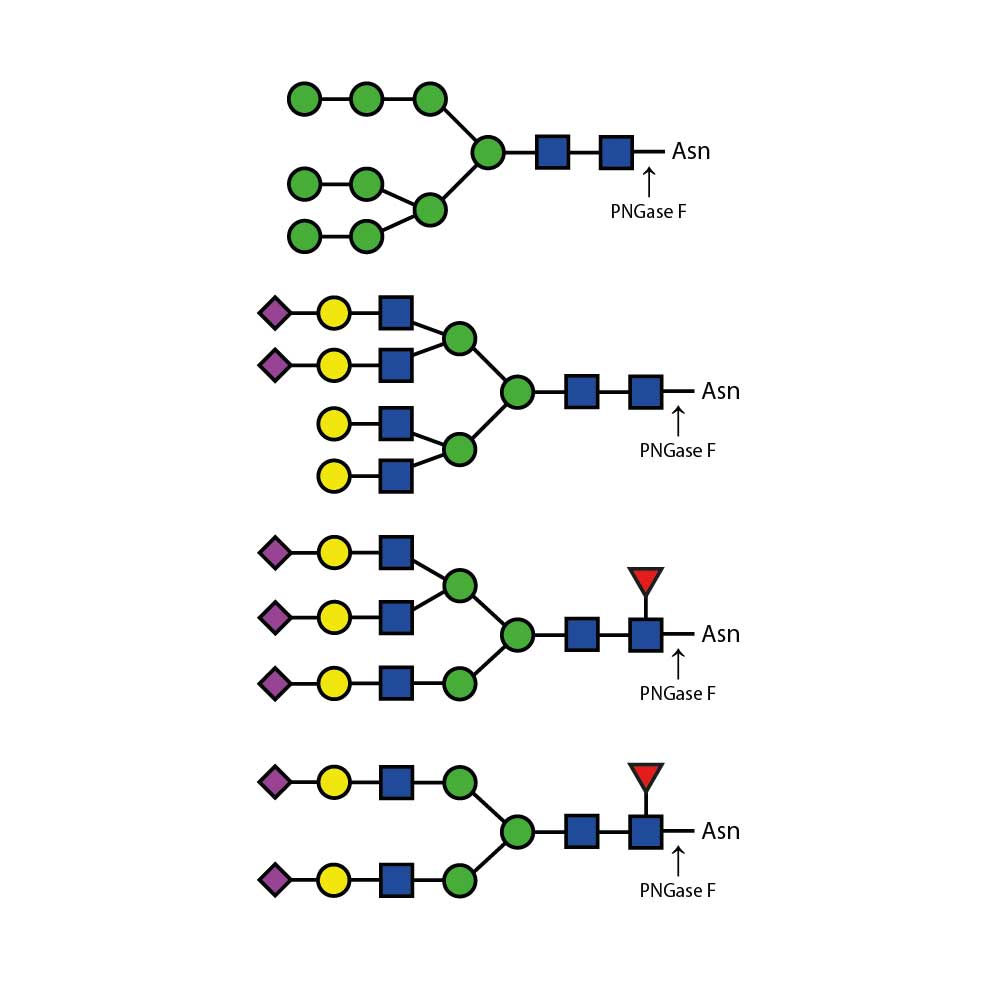
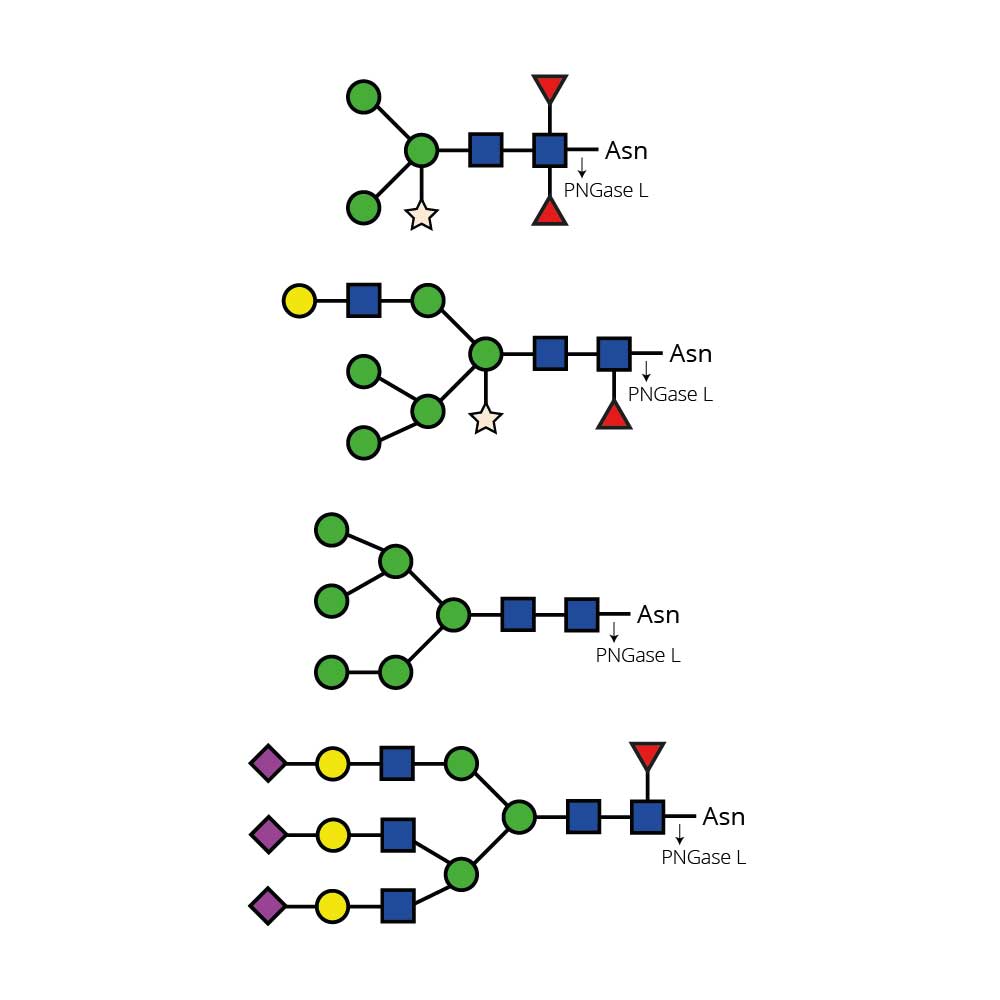
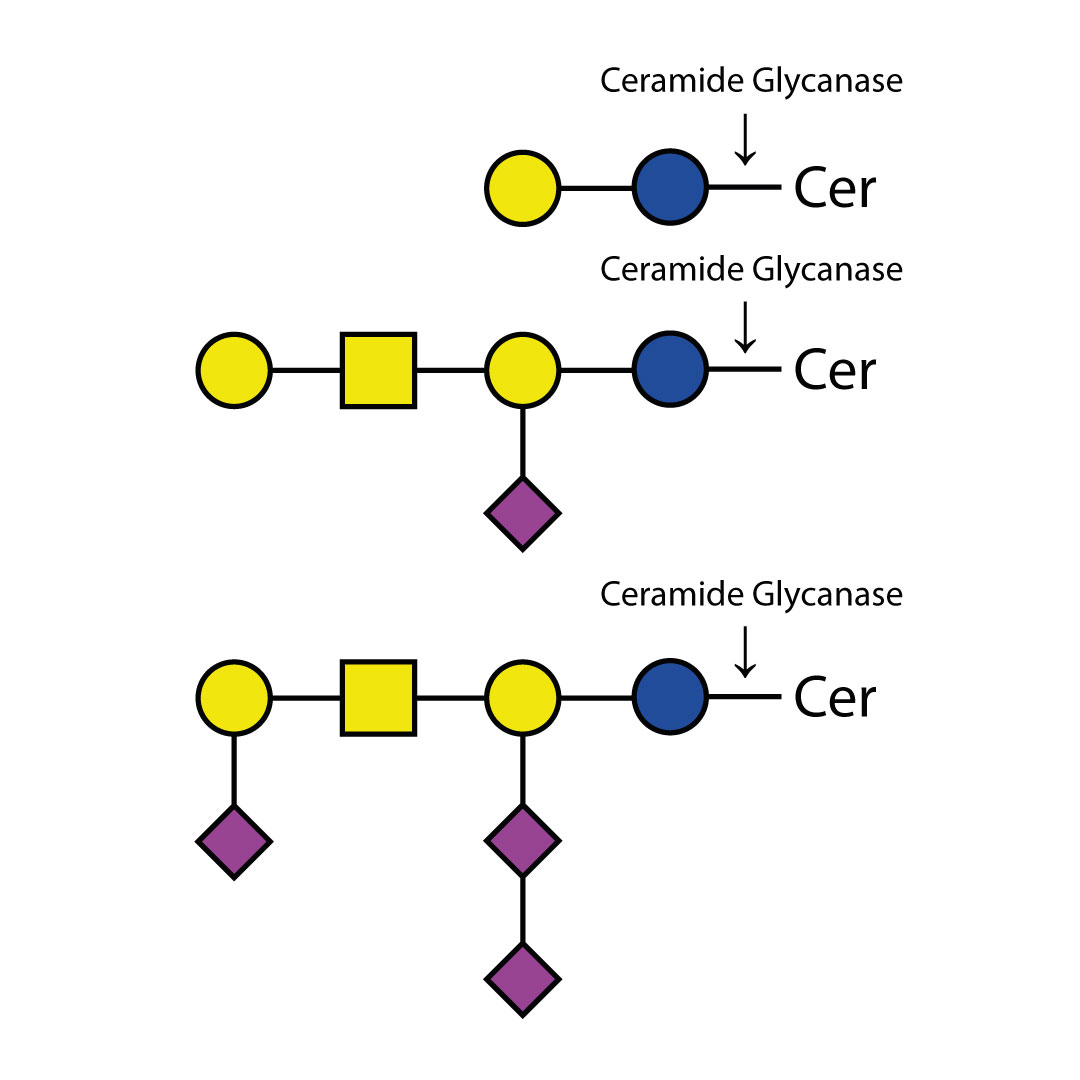
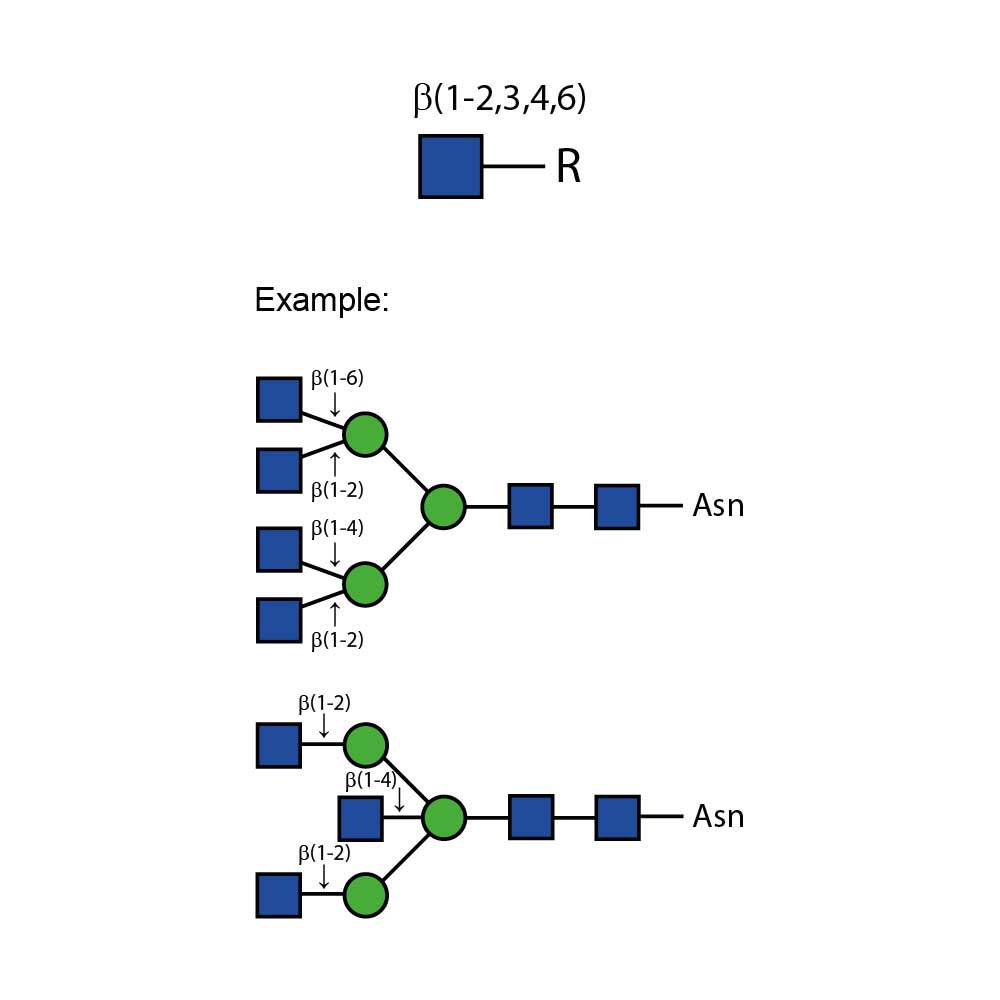
N - 乙酰葡萄糖胺苷酶可从复杂碳水化合物和糖蛋白中切除所有非还原末端β - 连接的N - 乙酰葡萄糖胺残基。
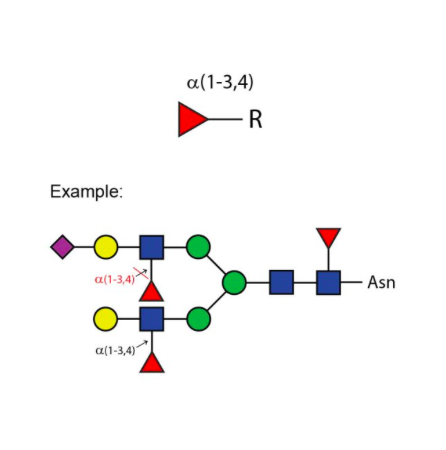
对于双天线、三天线和四天线寡糖中不同连接的GlcNAc(N - 乙酰葡萄糖胺)的切割速率,在很大程度上取决于相邻残基的空间位阻。对于所有这三种寡糖,连接于β(1 - 3) - 连接甘露糖的β(1 - 2)GlcNAc残基的切割速率最高,而连接于β(1 - 6) - 连接甘露糖的β(1 - 2)GlcNAc残基的切割速率最低。如果存在β(1 - 6)GlcNAc残基,其切除速率为第二高,β(1 - 4)GlcNAc残基则为第三。在三天线结构上,这种残基的切除速率为第二高。与β - 连接甘露糖相连的分支β(1 - 4)GlcNAc残基会严重阻碍其他GlcNAc残基的切割——需要高浓度的酶和较长的孵育时间才能实现切割 。
-
0.000.00
-
N - 乙酰葡萄糖胺苷酶可从复杂碳水化合物和糖蛋白中切除所有非还原末端β - 连接的N - 乙酰葡萄糖胺残基。
对于双天线、三天线和四天线寡糖中不同连接的GlcNAc(N - 乙酰葡萄糖胺)的切割速率,在很大程度上取决于相邻残基的空间位阻。对于所有这三种寡糖,连接于β(1 - 3) - 连接甘露糖的β(1 - 2)GlcNAc残基的切割速率最高,而连接于β(1 - 6) - 连接甘露糖的β(1 - 2)GlcNAc残基的切割速率最低。如果存在β(1 - 6)GlcNAc残基,其切除速率为第二高,β(1 - 4)GlcNAc残基则为第三。在三天线结构上,这种残基的切除速率为第二高。与β - 连接甘露糖相连的分支β(1 - 4)GlcNAc残基会严重阻碍其他GlcNAc残基的切割——需要高浓度的酶和较长的孵育时间才能实现切割 。
β - N - 乙酰葡萄糖胺苷酶的相关研究进展
一、β - N - 乙酰葡萄糖胺苷酶的发现
β - N - 乙酰葡萄糖胺苷酶(β - N - acetylglucosaminidase,GlcNAcase)的发现是糖苷酶研究领域逐步深入的结果。早期在对生物体内糖类代谢和细胞表面糖链结构的研究过程中,科学家们注意到存在一类能够特异性水解特定糖苷键的酶类。
随着生化技术和分子生物学的发展,研究人员通过对微生物、植物和动物组织进行酶活性测定和蛋白质纯化等工作,逐渐分离和鉴定出了β - N - 乙酰葡萄糖胺苷酶。最初,它可能是在研究某些微生物对复杂多糖的分解代谢时被发现的,之后又在多种生物来源中被检测到。进一步的研究确定了其具有水解β - N - 乙酰葡萄糖胺(GlcNAc)残基的特定功能,从而正式被命名为β - N - 乙酰葡萄糖胺苷酶。
二、对细胞功能的影响
(一)细胞黏附与迁移
细胞表面的糖基化修饰在细胞黏附和迁移过程中起着关键作用。β - N - 乙酰葡萄糖胺苷酶可以调节细胞表面糖蛋白和糖脂上的GlcNAc残基的水平,进而影响细胞与细胞外基质以及其他细胞之间的相互作用。例如,在肿瘤细胞的转移过程中,细胞表面糖基化模式的改变可能有助于肿瘤细胞脱离原发部位并迁移到其他组织。β - N - 乙酰葡萄糖胺苷酶通过改变细胞表面糖链的结构,参与了这一过程的调控。
(二)信号转导
细胞内的信号转导通路对于维持细胞的正常生理功能至关重要。糖基化修饰可以影响蛋白质的定位、稳定性和活性,从而参与信号转导过程。β - N - 乙酰葡萄糖胺苷酶通过调节糖基化水平,间接影响信号转导通路的激活或抑制。例如,某些受体蛋白的糖基化修饰可以影响其与配体的结合以及下游信号分子的招募,而β - N - 乙酰葡萄糖胺苷酶对受体蛋白糖基化的调节可以改变细胞的信号响应。
(三)细胞周期调控
细胞周期的正常运行对于细胞的生长、分裂和分化至关重要。研究发现,细胞周期进程与糖基化修饰密切相关。β - N - 乙酰葡萄糖胺苷酶可通过调节细胞周期相关蛋白的糖基化状态,影响细胞周期的各个阶段的转换。例如,在有丝分裂过程中,某些纺锤体组装相关蛋白的糖基化修饰可能受到β - N - 乙酰葡萄糖胺苷酶的调控,从而确保细胞分裂的准确性和稳定性。
三、与糖基化修饰的关系
(一)作为糖基化修饰的调控因子
β - N - 乙酰葡萄糖胺苷酶主要作用于β - 连接的N - 乙酰葡萄糖胺残基,通过水解这些残基来改变糖蛋白和糖脂的糖基化模式。在糖基化修饰过程中,GlcNAc残基可以作为其他糖基化修饰(如唾液酸化、岩藻糖化等)的基础,β - N - 乙酰葡萄糖胺苷酶对GlcNAc残基的去除会影响后续糖基化修饰的发生,从而精细调控糖链的结构和功能。
(二)参与糖基化的动态平衡
细胞内的糖基化修饰是一个动态的过程,涉及到糖基的添加和去除。β - N - 乙酰葡萄糖胺苷酶与负责糖基添加的酶(如糖基转移酶)共同维持着糖基化的动态平衡。在不同的生理条件和细胞状态下,β - N - 乙酰葡萄糖胺苷酶的活性会发生变化,从而调节糖基化的水平和模式,以适应细胞的需求。
四、在糖尿病诊断和治疗中的应用
(一)糖尿病诊断方面
- **生物标志物**:糖尿病患者体内的糖代谢紊乱可导致细胞表面糖基化修饰的改变,β - N - 乙酰葡萄糖胺苷酶的活性或相关糖基化产物的水平可成为糖尿病诊断的新型生物标志物。例如,研究发现糖尿病患者血液或尿液中某些糖蛋白的糖基化模式发生了变化,其中β - N - 乙酰葡萄糖胺苷酶参与调控的糖基化位点的变化与糖尿病的发生和发展密切相关。通过检测这些生物标志物的变化,可以实现糖尿病的早期诊断和病情监测。
- **代谢组学研究**:代谢组学技术可以对生物体内的小分子代谢物进行全面分析。β - N - 乙酰葡萄糖胺苷酶参与的糖基化修饰过程产生的代谢产物可以通过代谢组学方法进行检测和分析。通过比较糖尿病患者和健康人群的代谢组学数据,寻找与β - N - 乙酰葡萄糖胺苷酶相关的特征性代谢产物,有助于深入了解糖尿病的发病机制,并为诊断提供新的线索。
(二)糖尿病治疗方面
- **药物研发靶点**:基于β - N - 乙酰葡萄糖胺苷酶在糖基化修饰中的重要作用,它可以作为糖尿病治疗的潜在药物靶点。开发能够调节β - N - 乙酰葡萄糖胺苷酶活性或表达的药物,有望改善糖尿病患者的糖代谢紊乱状况。例如,设计特异性的β - N - 乙酰葡萄糖胺苷酶抑制剂,通过调节细胞表面糖基化模式,改善胰岛素信号转导和葡萄糖摄取,从而达到降低血糖的目的。
- **基因治疗策略**:随着基因治疗技术的发展,针对β - N - 乙酰葡萄糖胺苷酶的基因治疗方法也逐渐受到关注。通过基因编辑技术(如CRISPR/Cas9)对编码β - N - 乙酰葡萄糖胺苷酶的基因进行修饰,调节其表达水平或活性,可能为糖尿病的治疗提供新的策略。例如,在糖尿病患者体内过表达具有特定功能的β - N - 乙酰葡萄糖胺苷酶变体,以纠正异常的糖基化修饰,改善细胞功能。
产品说明书:
https://www.qa-bio.com/docs/E-GL01.QA-Bio.specsheet.pdf
产品质量规格
来源:在大肠杆菌中重组表达自肺炎链球菌。
酶学分类编号(EC):3.2.1.52
其他名称:β - N - 乙酰葡萄糖胺苷酶、N - 乙酰 - β - D - 糖苷胺N - 乙酰葡萄糖胺水解酶、葡萄糖胺苷酶、己糖胺苷酶
试剂盒包含:
Ludger β - (1 - 2,3,4,6) - N - 乙酰葡萄糖胺苷酶
含N - 乙酰葡萄糖胺苷酶的溶液,溶剂为20 mM Tris - HCl,25 mM NaCl,pH值7.5
5倍反应缓冲液250 mM磷酸钠,pH值5.0
比活性:>80 U/mg
活性:>50 U/mL
分子量:约140,000道尔顿
pH范围:5 - 7,最适pH为5.0
建议用法:
1. 向试管中加入最多100微克的糖蛋白或1纳摩尔的寡糖。
2. 用去离子水定容至最终体积14微升。
3. 加入4微升5倍反应缓冲液(pH值5.0)。
4. 加入2微升N - 乙酰葡萄糖胺苷酶。
5. 在37°C下孵育3小时。
注:如果存在平分型GlcNAc或β(1 - 2)GlcNAc - α(1 - 6)Man,将孵育时间延长至18小时。
特异性:切割所有非还原末端β - 连接的N - 乙酰葡萄糖胺。平分型GlcNAc会减慢反应速度。
比活性测定:定义为在37°C、pH值5.0条件下,1分钟内从对硝基苯酚 - β - D - N - 乙酰 - 葡萄糖胺苷产生1微摩尔对硝基苯酚(pNP)所需的酶量。
储存:将酶储存于4°C。
纯度:每一批次的N - 乙酰葡萄糖胺苷酶均通过如下方法检测是否存在污染性蛋白酶:将10微克变性的牛血清白蛋白(BSA)与2微升酶在24小时内孵育。经十二烷基硫酸钠 - 聚丙烯酰胺凝胶电泳(SDS - PAGE)分析处理后的BSA,无降解迹象。
生产宿主菌株已进行广泛测试,未检测到产生任何可检测的糖苷酶。
稳定性:在适当储存条件下至少稳定12个月。短时间暴露于室温不会降低活性 。
电话:186 0210 8329
157 1167 5909
cindy.li@ludger.com
QQ:258363672 170439096
Ludger

QQ(Lily): 258363 672 QQ(Cindy): 170 439 096
地 址:上海静安区南京西路1266号